Ethylene glycol is a 1,2glycol compound produced via reaction of ethylene oxide with waterIt has a role as a metabolite, a toxin, a solvent and a mouse metabolite It is a glycol and an ethanediolFind SigmaAldrichP MSDS, related peerreviewed papers, technical documents, similar products & more at SigmaAldrich1,1Ethanediol, diacetate Formula C 6 H 10 O 4 Molecular weight IUPAC Standard InChI InChI=1S/C6H10O4/c14 (7)96 (3)105 (2)8/h6H,13H3 Copy Sheet of paper on top of

1 2 Ethanediol Glendry Anhydrous Cas 107 21 1 Glentham Life Sciences
1 1 ethandiol
1 1 ethandiol-Noun 1 ethanediol a sweet but poisonous syrupy liquid used as an antifreeze and solvent The chemical equilibrium between formaldehyde (HCHO) and its hydrated form, methanediol (CH 2 (OH) 2), is computationally investigated in solvent waterUsing the method of energy representation, the solvation free energy of methanediol is evaluated as a function of (solvent) density and temperature, and the solvent effect is discussed over a wide range of




S S 1 2 Diphenyl 1 2 Ethanediol 98 Acros Organics Fisher Scientific
ChemBlink provides information about CAS # , 1,2Ethanedithiol, 1,2Dimercaptoethane, Dithioethyleneglycol, Ethylene mercaptan, molecular formula C2H6S21,1Ethanediol Diacetate Predicted ACD/Labs Predicted ChemAxon Predicted data is generated using the ACD/Labs Percepta Platform PhysChem Module No predicted properties have been calculated for this compound Density Boiling1,2Dihydro1,5dimethyl2phenyl3Hpyrazol3one compound with 2,2,2trichloro1,1ethanediol (12), Dichloralantipyrine
2,2,2Trifluoro1,1ethanediol (contains Total ca 25% Water) Product # T3279 CAS RN Purity / Analysis Method View Full Details Product Categories Chemistry Building Blocks Fluorinated Building Blocks NonHeterocyclic Fluorinated Building Blocks Toxicology and Carcinogenesis Studies of Chloral Hydrate (CASRN ) in B6C3F1 Mice (Gavage Studies) Mice MV Carcinogenesis Results Female Mice Equivocal Evidence Dose 0, 25, 50, OR 100 MG/KG 2 years (Gavage) (C910C) Completed TR503 (NIH Number ) (Peer Review Approval A )Fig1 Logarithmic plot of the experimental concentrations x (mol dm3 ) of free formaldehyde at its total concentration c 0 = 10 mol dm3 and 395 ± 3 K in ( ) water, ( ) methanol, ( ) ethanol
ATKXDQOHNICLQWUHFFFAOYSAN Dichloralphenazone USPINNBAN Similar structures search, synonyms, formulas, resource links, and other chemical information1,1Ethanediol diacetate ≥99% 1,1Ethanediol diacetate Formula C₆H₁₀O₄ Boiling Pt 69 °C (17 mmHg) Flash Pt 68 °C Storage Temperature Ambient MDL Number MFCD CAS Number1,1Ethanediol, diacetate none listed none listed none listed OSHA Vacated PELs 1,1Ethanediol, diacetate No OSHA Vacated PELs are listed for this chemical Personal Protective Equipment Eyes Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR or
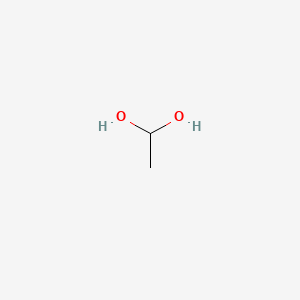



1 1 Ethanediol C2h6o2 Pubchem



Density Of 1 2 Ethanediol From Dortmund Data Bank
1,1Ethanediol diacetate ≥99% Supplier Acros Organics Description Size 100GM CAS Number SDS ,EA,EA,EA This product is no longer available Alternatives may be available by searching with the VWR Catalog Number listed above If you need further assistance, please call VWR Customer Service at 1Shop Ethylidene diacetate, 99%, ACROS Organics™ at FisherscicomEthylene glycol is an organic compound with the formula (CH2OH)2 It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations It is an odorless, colorless, sweettasting, viscous liquid
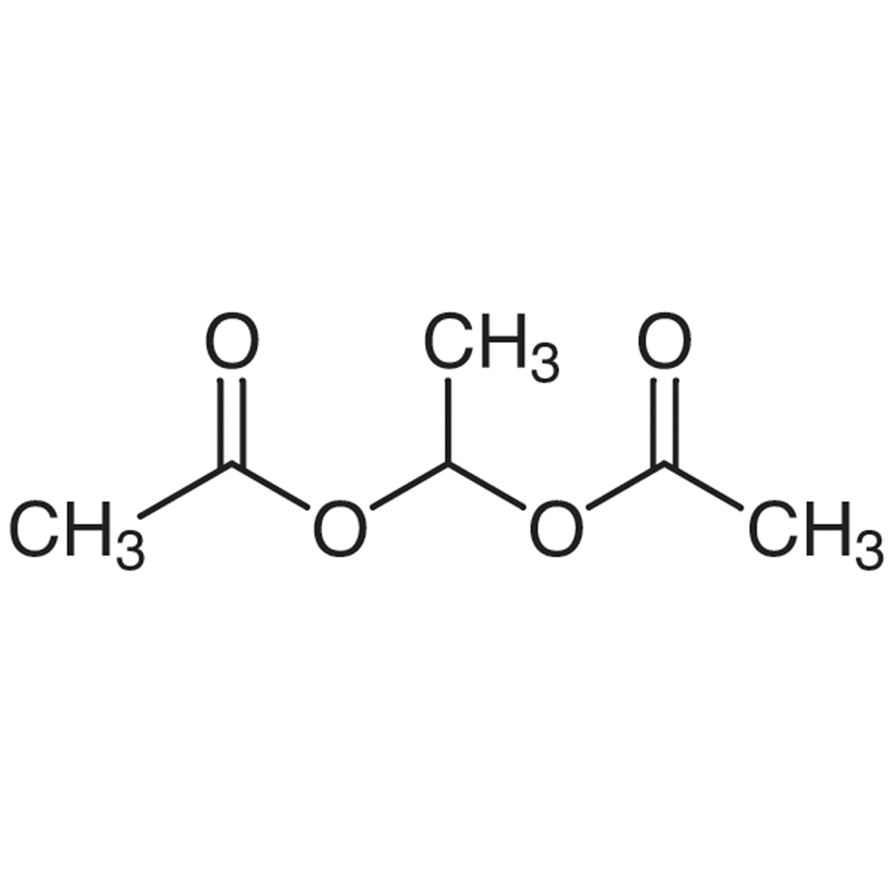



1 1 Ethanediol Diacetate 542 10 9 3b E0030 Cymit Quimica




1 1 Ethanediol Diacetate 98 0 Tci America Fisher Scientific
Acetaldehyde, hydrate Molecular Structure Molecular Formula C 2 H 6 O 2 Molecular Weight 67 CAS Registry Number Compound 1,1Ethanediol, diacetatewith free spectra 12 NMR, 2 FTIR, and 7 MS1,1Ethanediol, 2,2,2trifluoro Unknown Inactive EPA Applications/Systems Below are the EPA applications/systems, statutes/regulations, or other sources that track or regulate this substance This table shows how each list refers to the substance To view more metadata about the specific Synonym, click on the Synonym
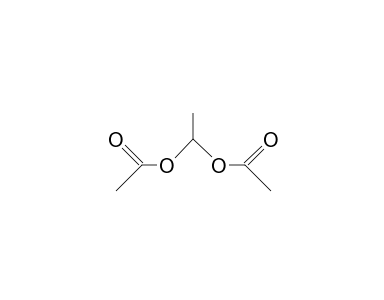



1 1 Ethanediol Diacetate 13c Nmr Chemical Shifts Spectrabase



1 1 Ethanediol 2 Phenyl Diacetate
AZHSSKPUVBVXLKUHFFFAOYSAN 1,1Ethanediol Similar structures search, synonyms, formulas, resource links, and other chemical informationMfr Model # EG UNSPSC # Catalog Page # N/A Country of Origin USA Country of Origin is subject to change Compare this product Web PriceSomnos Chloral hydrate is used medically as a sedative or hypnotic and as a rubefacient in topical preparations, and it is often given to children as a sedative during dental and other medical procedures




S S 1 2 Diphenyl 1 2 Ethanediol 98 Acros Organics Fisher Scientific
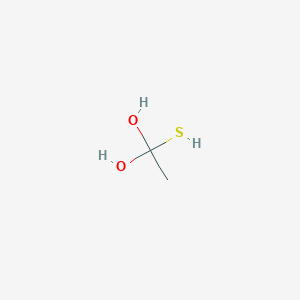



1 Mercapto 1 1 Ethanediol C2h6o2s Pubchem
2,2,2trichloro1,1ethanediol Trade names Aquachloral Supprettes;IUPAC Standard InChIKey LYCAIKOWRPUZTNUHFFFAOYSAN Copy CAS Registry Number Chemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Species with the same structure1,1Ethanediol Diacetate textskipToContent textskipToNavigation TCI uses cookies to personalize and improve your user experience By continuing on our website, you accept the use of cookies You can change or update your cookiesettings at any time Accept




The 10 Symmetry Distinct Rotamers Of 1 2 Ethanediol Depicted As Newman Download Scientific Diagram
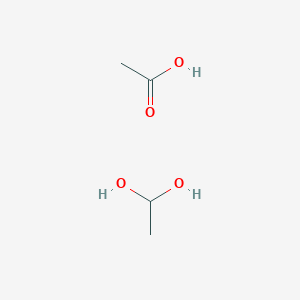



1 1 Ethanediol Monoacetate C4h10o4 Pubchem
1,1Ethanediol, 2,2,2trichloro Chloral hydrate » Chloral Hydrate contains not less than 995 percent and not more than 1025 percent of C 2 H 3 Cl 3 O 2Molecular Weight Molecular Formula C 3 H 8 O 3;Brief Method Summary A 50mL sample is extracted with 3mL of MTBE or 5mL of pentane The concentrations of compounds in the extract are measured using a fused silica capillary column gas chromatography (GC) system equipped a linearized electron capture detector (ECD) Scope and




1 1 Ethanediol Structure C2h6o2 Over 100 Million Chemical Compounds Mol Instincts




1 2 Ethanediol Diformate Cas 629 15 2 Chemical Physical Properties By Chemeo
Chemsrc provides 1,1Ethanediol, 2phenyl, 1,1diacetate(CAS#8037) MSDS, density, melting point, boiling point, structure, formula, molecular weight etcOne general application of trifluoroacetaldehyde is the preparation of trifluoroethylamino derivatives via reductive amination reaction This synthesis includes the formation of the corresponding N,Oacetal intermediates followed by their reduction using NaBH4 or 2picoline borane complex, affording the target trifluoroethylamino compounds in moderate to good yieldsNames and Identifiers Properties Safety and Handling SDS SAFETY DATA SHEETS According to Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Sixth revised edition




891 19 9 1 1 Ethanediol 2 2 2 Trichloro Mixt With 1 2 Ethanediol C4h9cl3o4 Formula Nmr Boiling Point Density Flash Point




1 1 Ethanediol 2 2 2 Trifluoro 1 4 Methoxyphenyl Cas 8 Chemical Physical Properties By Chemeo
Chemsrc provides ethane1,1diol(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of ethane1,1The 2D chemical structure image of 1,1Ethanediol, 2,2,2trichloro1phenyl is also called skeletal formula, which is the standard notation for organic moleculesFile1,1ethanediolsvg Size of this PNG preview of this SVG file 170 × 109 pixels Other resolutions 3 × 5 pixels 640 × 410 pixels 800 × 513 pixels 1,024 × 657 pixels 1,280 × 1 pixels 2,560 × 1,641 pixels This is a file from the Wikimedia Commons Information from its description page there is shown below




S 1 Phenyl 1 2 Ethanediol 99 Acros Organics Fisher Scientific




1 2 Ethanediol Monoacetate Cas 542 59 6 Odour Threshold Value
1,1Ethanediol List of Suppliers Currently there are no suppilers available for 1,1Ethanediol Welcome to suggest suppliers to chemBlink Identification Name 1,1Ethanediol Synonyms 1,1Dihydroxyethane;Y\files\classes\424\lab info & exps\Exp 3 diol oxidation studentsDOC Oxidation is an important process in chemistry Of course, with any oxidation there must also be reduction,1, 1Ethanediol Diacetate, 25g Item # 49E6;



1 1 Ethanediol Diacetate




Ethanediol An Overview Sciencedirect Topics
View entire compound with free spectra 12 NMR, 2 FTIR, and 7 MS SpectraBase Compound ID 87qSiU0TC InChI InChI=1S/C6H10O4/c14 (7)96 (3)105 (2)8/h6H,13H3 InChIKey ACKALUBLCWJVNBUHFFFAOYSAN Google Search Mol Weight g/molCHLORAL HYDRATE is incompatible with alkalis, alkaline earth metals, alkali carbonates and soluble barbiturates It is decomposed by sodium hydroxide It reduces ammoniacal silver nitrate It liquefies when triturated with an equal quantity of menthol, camphor or thymol (NTP, 1992)Substance Details CAS Registry Number CA Index Name 1,1Ethanediol, 2,2,2trichloro
/2F02563C4EE712FB802586AF00419D02/$file/AAA54210_structure.png)



1 1 Ethanediol Diacetate 542 10 9 Biosynth Carbosynth Product
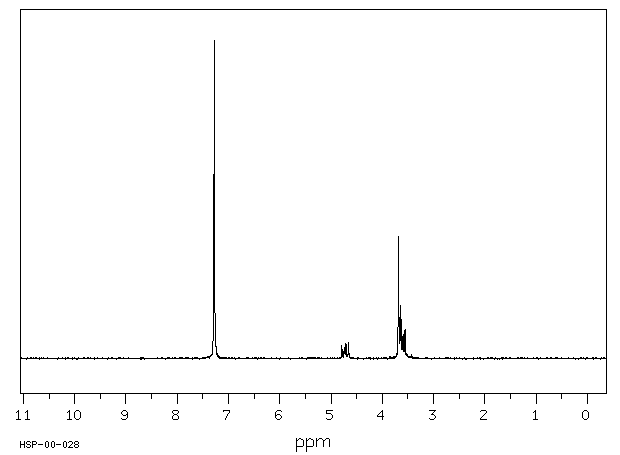



1 Phenyl 1 2 Ethanediol 93 56 1 1h Nmr
For a linear molecule, the rotational partition function is McQuarrie, x46, Eq 438 q r = 1 ˙ r T r where r = h 2=8ˇIk BI is the moment of inertia The rotational contribution to the First, 1,1ethanediol is a hydrate of ethanal (acetaldehyde) which is produced by adding water to ethanal The reaction occurs in either acidic or basic media and is reversible The means that an attempt to isolate the 1,1ethanediol from the solution leads to the recovery of ethanal and waterHydrate, Chloral SNOMED CT Chloral hydratecontaining product ();
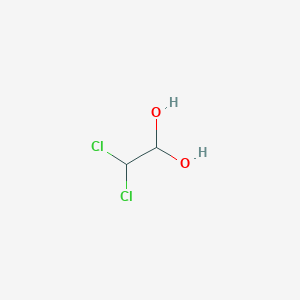



2 2 Dichloro 1 1 Ethanediol C2h4cl2o2 Pubchem




File 1 1 Ethanediol Png Wikimedia Commons
1,1Ethanediol, 1methoxy (CAS No ) SDS CAS No ;1,1Ethanediol, diacetate Valid Additional Synonyms There are no Synonyms of this type Top of Page Related Substances This substance is not a Related Substance of any other substance Top of Page Health and Other Scientific InformationEthylene Glycol (also 1,2ethanediol), HOCH2 CH 2OH, the simplest of the glycols Ethylene glycol takes the form of a colorless, viscous liquid with a sweet taste It has a melting point of – 123°C, a boiling point of 196°C, and a density of 1113 g/cm3 at °C Miscible in all proportions with water, alcohol, and acetone, it is poorly




Buy Big Discount Purity 99 2 2 2 Trichloro 1 1 Ethanediol 302 17 0 With Best Quality Industrial Grade 99 From Shanghai Yunao International Trade Co Ltd




Alfa Aesar S 1 1 2 Triphenyl 1 2 Ethanediol 98 Fisher Scientific
Ethane1,2diol (Ethylene glycol) Ethane1,2diol, (ethylene glycol, monoethylene glycol, MEG) which is manufactured from ethene via epoxyethane, is used to make polyester fibres, resins and films although it is probably better known for its use as a coolant in cars It is miscible with water and it lowers the freezing point of water so it isThe chemical equilibrium between formaldehyde (HCHO) and its hydrated form, methanediol (CH 2 (OH) 2), is computationally investigated in solvent waterUsing the method of energy representation, the solvation free energy of methanediol is evaluated as a function of (solvent) density and temperature, and the solvent effect is discussed over a wide range ofThe 2D chemical structure image of 1,1Ethanediol is also called skeletal formula, which is the standard notation for organic molecules The carbon atoms in the chemical structure of 1,1Ethanediol are implied to be located at the corner(s) and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is considered to be associated with enough hydrogen




1 1 Ethanediol Semantic Scholar
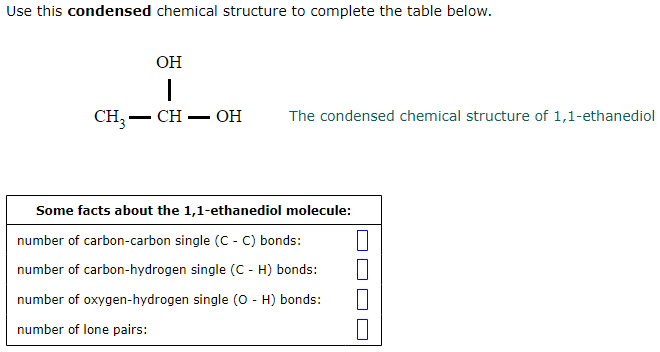



Solved Use This Condensed Chemical Structure To Complete The Chegg Com
small molecules, and methanediol has extreme stability in isolation 21–24 1,1Ethanediol in the gas phase has not been studied experimentally, but its reactions are probably similar to methanediol Once we accept the gasphase existence of these molecules, we also have to assume that they cross the air–water interface in the diol form




1r 2r 1 2 Dicyclohexyl 1 2 Ethanediol 95 1850 92 2



1 1 Ethanediol C2h6o2 Md Topology Nmr X Ray




1 2 Ethanediol Diacetate Cas 111 55 7 Chemical Physical Properties By Chemeo




1 1 2 2 Tetraphenyl 1 2 Ethanediol Cas 464 72 2 Scbt Santa Cruz Biotechnology



1 3 Propanediol 1 2 Ethanediol 1 1 C5h14o4 Chemspider




1 1 2 2 Tetrakis 4 Methylphenyl 1 2 Ethanediol 913 86 0 1h Nmr




China Big Discount Purity 99 2 2 2 Trichloro 1 1 Ethanediol 302 17 0 With Best Quality China 302 17 0 Cas 302 17 0
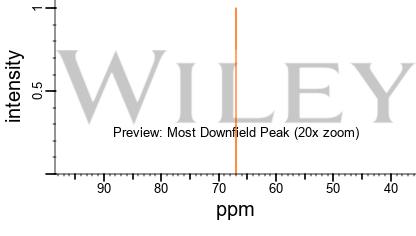



1 1 Ethanediol 17o Nmr Chemical Shifts Spectrabase



Pqr 1 1 Ethanediol




1 2 Ethanediol Glendry Anhydrous Cas 107 21 1 Glentham Life Sciences



1



1 2 Ethanediol 1 2 Diphenyl R R




Alcohols And Ethers Chemistry Atoms First




S 1 1 2 Triphenyl 1 2 Ethanediol 1098 0 Tci America



Meso 1 2 Diphenyl 1 2 Ethanediol Supplier Casno 579 43 1



1 1 Ethanediol 1 2 Amino 5 Chlorophenyl 2 2 2 Trifluoro Hydrochloride 1



1 1 Ethanediol C2h6o2 Md Topology Nmr X Ray
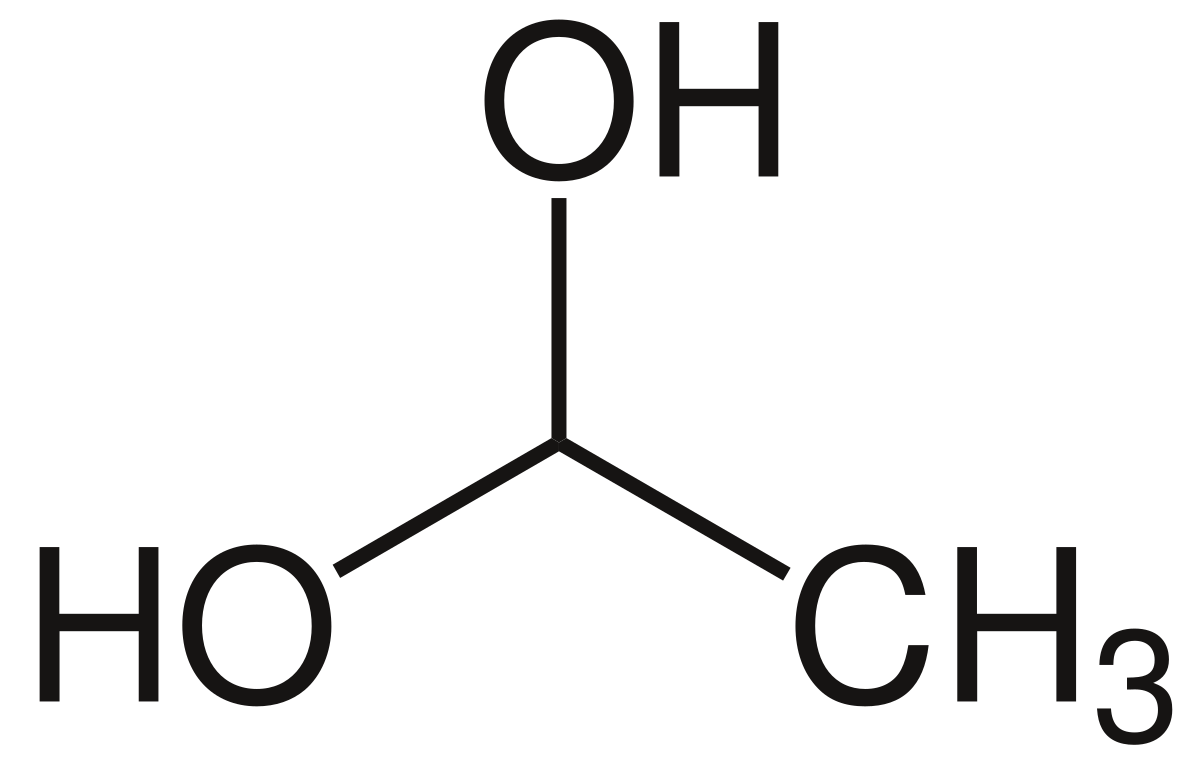



File 1 1 Ethanediol Svg Wikipedia
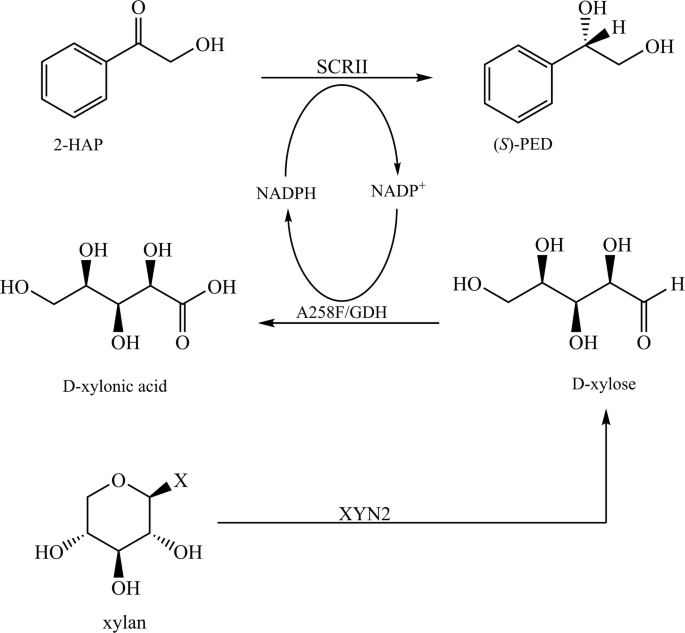



Efficient Production Of S 1 Phenyl 1 2 Ethanediol Using Xylan As Co Substrate By A Coupled Multi Enzyme Escherichia Coli System Microbial Cell Factories Full Text
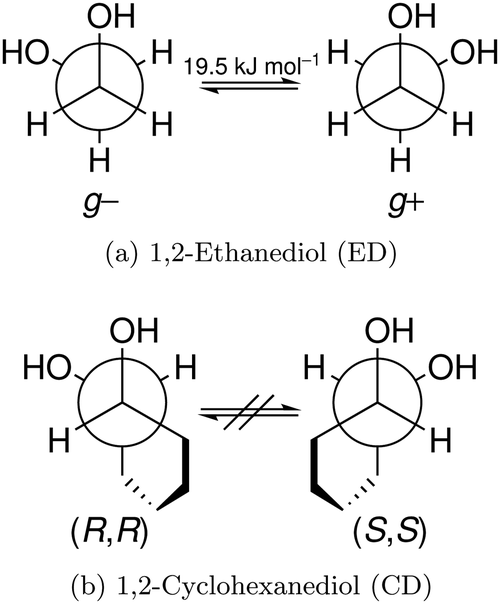



The Reduced Cohesion Of Homoconfigurational 1 2 Diols Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C9cpf



1
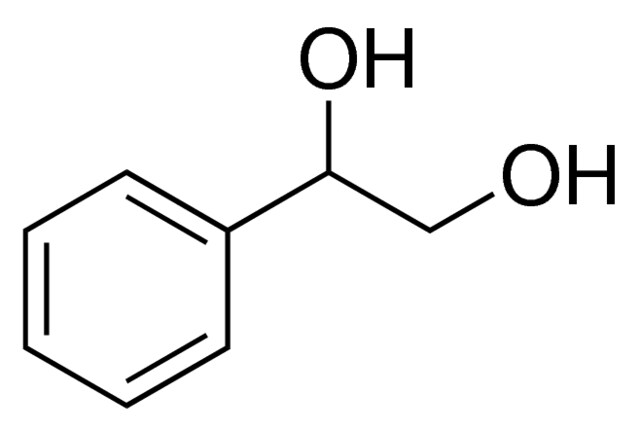



1 Phenyl 1 2 Ethanediol 97 93 56 1




1 1 Ethanediol 2 2 2 Trifluoro 421 53 4 Purity 96 United States




1 2 Ethanediol




1 1 2 2 Tetraphenyl 1 2 Ethanediol 99 464 72 2




File 1 1 Ethanediol Svg Wikipedia
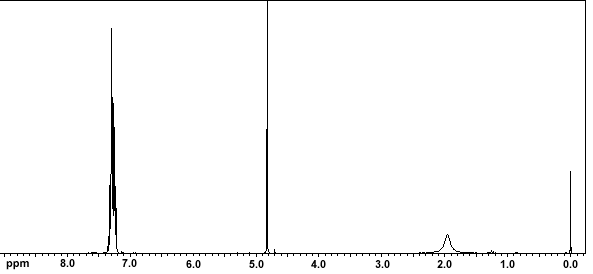



Nop Sustainability In The Organic Chemistry Lab Course




Association Of The Pyridine 1 2 Ethanediol Complexes Download Scientific Diagram



Is 1 2 Ethanediol Polar Mcat



Human Metabolome Database Showing Metabocard For 2 2 Dichloro 1 1 Ethanediol Hmdb
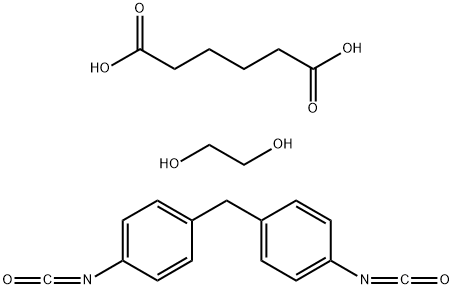



01 5 Cas Msds Hexanedioic Acid Polymer With 1 2 Ethanediol And 1 1 Methylenebis4 Isocyanatobenzene Melting Point Boiling Point Density Cas Chemical Properties




1 Phenyl 1 2 Ethanediol 93 56 1 C8h10o2 Density Melting Point Boiling Point Structural Formula Synthesis




New Methods To Obtain Carboxylic Acids By Oxidation Reactions Of 1 2 Ethanediol With Metallic Nitrates Sciencedirect
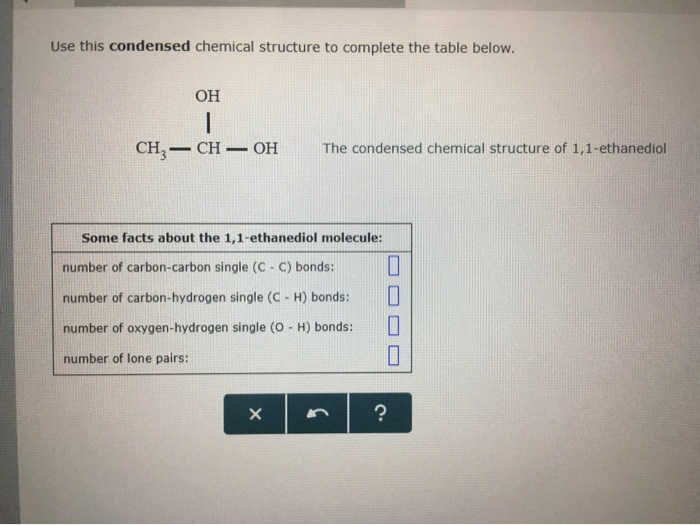



Solved Use This Condensed Chemical Structure To Complete The Chegg Com
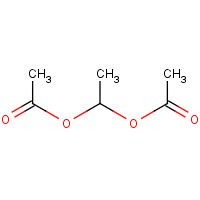



1 1 Ethanediol Diacetate 542 10 9 54 Or9351 Cymitquimica
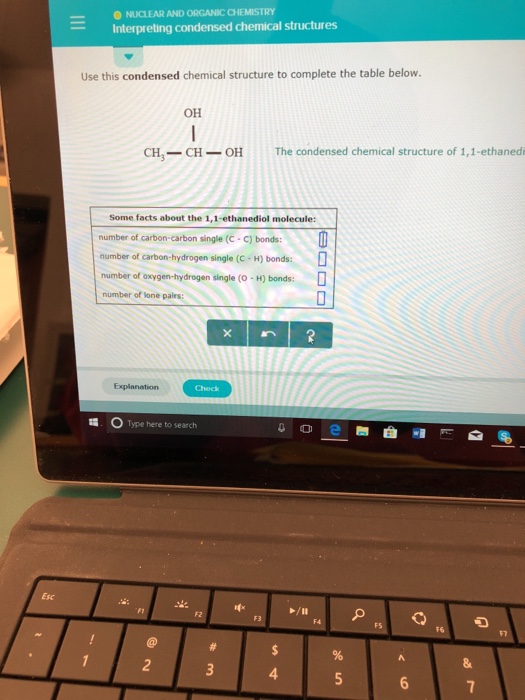



Solved Interpreting Condensed Chemical Structures Use This Chegg Com
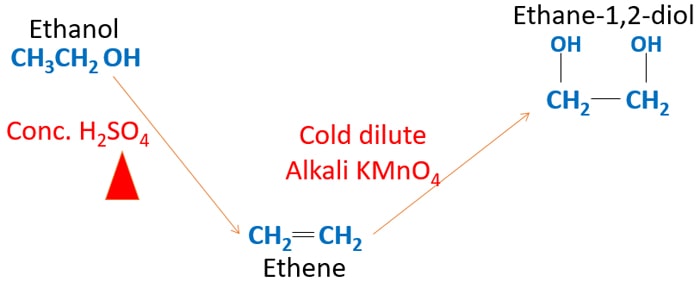



Ethanol To Ethane 1 2 Diol Ethylene Glycol 1 2 Ethanediol
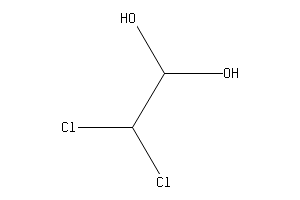



2 2 Dichloro 1 1 Ethanediol Chemical Substance Information J Global



1 2 Ethanediol Sodium Salt 1 1 C2h6nao2 Chemspider



2 2 2 Trichloro 1 1 Ethanediol
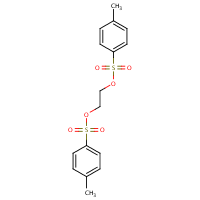



1 2 Ethanediol Bis 4 Methylbenzenesulfonate Hazardous Agents Haz Map




1 2 Bis 4 Methoxyphenyl 1 2 Ethanediol Aldrichcpr




R 1 1 2 Triphenyl 1 2 Ethanediol Cas 46 4 Scbt Santa Cruz Biotechnology



1



Meso 1 2 Diphenyl 1 2 Ethanediol Cas 492 70 6 0526




R 1 Phenyl 1 2 Ethanediol Cas 00 3 Scbt Santa Cruz Biotechnology




99 2 2 Bromo 2 Chloro 1 1 Ethanediol 90 2 Bromo 2 Chloroethane 1 1 Diol C H Brclo Trc
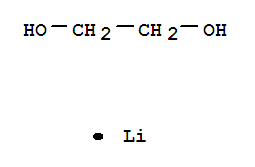



1 2 Ethanediol Lithium Salt 1 1 Supplier Casno 23 9



56 4 1 1 Ethanediol 2 2 2 Trichloro Diacetate Cas No 56 4 1 1 Ethanediol 2 2 2 Trichloro Diacetate
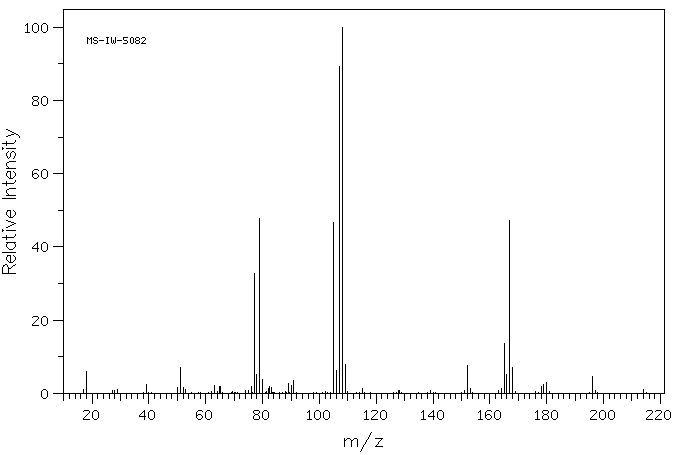



Meso 1 2 Diphenyl 1 2 Ethanediol 579 43 1 Ms



1 1 2 Triphenyl 1 2 Ethanediol Regis Technologies




Structures Of 1 Phenyl 1 2 Ethanediol A Biphenyl 4 4 Diol B Download Scientific Diagram




579 43 1 R S Dihydrobenzoin Rel 1r 2s 1 2 Diphenyl 1 2 Ethanediol 1r 2s 1 2 Diphenyl 1 2 Ethanediol Nsc Cis 1 2 Diphenyl 1 2 Ethanediol Erythro 1 2 Diphenylethane 1 2 Diol Erythro Hydrobenzoin Meso 1 2 Diphenyl 1 2 Ethanediol




S 1 Phenyl 1 2 Ethanediol 97 Vwr



1 1 Ethanediol Diisobutyrate



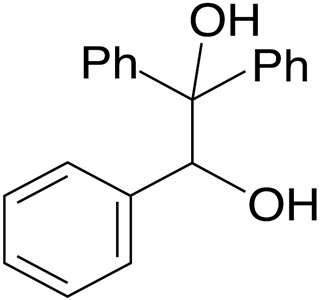
Water Ethanediol C2h8o3 Pubchem




1 2 Nitrophenyl 1 2 Ethanediol 98 59 7



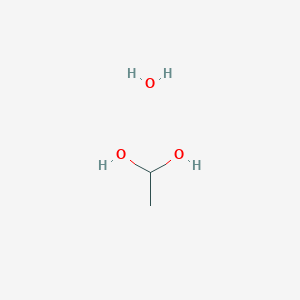
1 Mercapto 1 1 Ethanediol Chemical Substance Information J Global




Acros Organics Ethylene Glycol 99 25l Cas 107 21 1 1 2 Ethanediol 1 2 Ethanediol From Masterflex




Ethanediol An Overview Sciencedirect Topics



1 1 Ethanediol Acetic Acid 1 2 C6h14o6 Chemspider




1 2 Ethanediol Dipropanoate Cas 123 80 8 Chemical Physical Properties By Chemeo



1 1 Ethanediol C2h6o2 Chemspider



1 2 Ethanediol Methoxyethene 1 1 C5h12o3 Chemspider
/0E73E5FFC846BA69802585F9006A1A34/$file/FE76275_structure.png)



1 2 Ethanediol Ditosylate 6315 52 2 Biosynth Carbosynth




2 2 2 Trifluoro 1 1 Ethanediol 421 53 4 Tci America




Highly Enantioselective Deracemization Of 1 Phenyl 1 2 Ethanediol And Its Derivatives By Stereoinversion Using Candida Albicans In A One Pot Process Sciencedirect




1 Amino 1 2 Ethanediol Molecular Weight C2h7no2 Over 100 Million Chemical Compounds Mol Instincts



542 10 9 1 1 Ethanediol Diacetate Tetrahedron



2 2 2 Trichloro 1 1 Ethanediol 1 2 Ethanediol 1 1 C4h9cl3o4 Chemspider
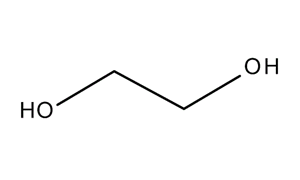



107 21 1 Cas Ethanediol Alcohols Article No




1 1 Ethanediol Diacetate Spectrabase




1 1 Ethanediol Semantic Scholar



Ethanediol




R 1 Phenyl 1 2 Ethanediol 98 Frontier Specialty Chemicals
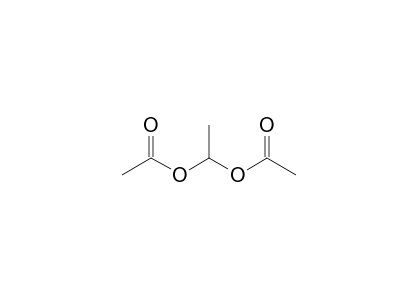



1 1 Ethanediol Diacetate 1h Nmr Chemical Shifts Spectrabase
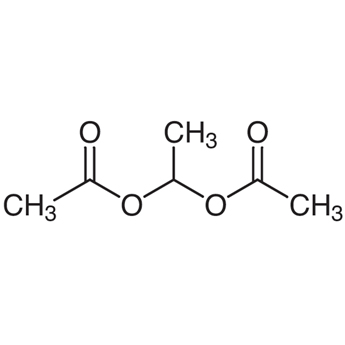



1 1 Ethanediol Diacetate 542 10 9 Tci Shanghai Development Co Ltd



1 1 Ethanediol Lone Pairs


